Phone:
(+212)6814-6992
Physical address:
Cardioplus, Green street, Casablanca, Morocco.



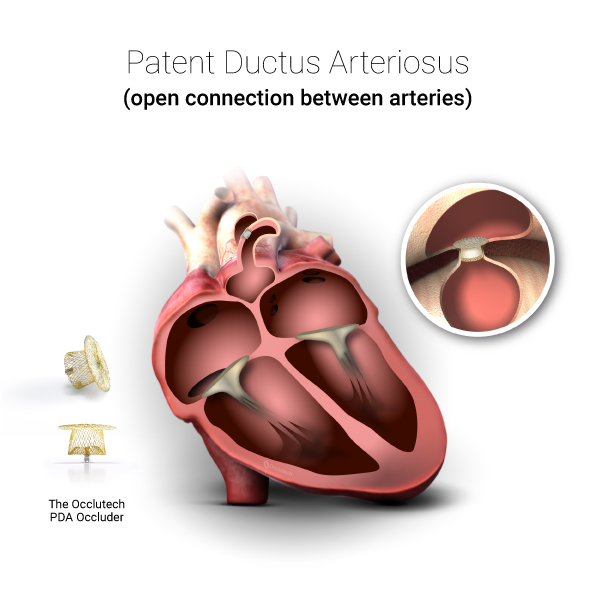



Occlutech provides both regular shank and long shank PDA occluders to address different types of defects.
Key Features and Benefits
Advantages
The Occlutech PDA Occluder offers several significant advantages:
Clinical Design and Efficacy
Occlutech PDA Occluder Specifications
Occlutech provides a range of sizes for both regular and hubless PDA occluders to ensure optimal fit and performance:
Patent Ductus Arteriosus (PDA) is a congenital heart defect characterized by the persistence of the ductus arteriosus, a vessel that connects the pulmonary artery to the descending aorta, which normally closes shortly after birth. PDAs represent 5% to 10% of all congenital heart defects. The overall incidence of symptomatic PDA is 1 in 2000, while the total incidence, including “silent” PDAs, can reach up to 1 in 500. Approximately 5,200 new symptomatic PDA cases are diagnosed annually in the United States, Europe, and Japan, with around 40,000 interventions performed each year, predominantly in developing countries.
Types of PDA
PDAs occur with various morphologies, which can influence the choice of treatment. Occlutech offers two different designs with different attachment mechanisms to accommodate these variations.