Key Features
- Paclitaxel-Coated Balloon: Delivers targeted drug therapy to inhibit restenosis and promote long-term vessel patency.
- Controlled Drug Delivery: Ensures consistent and controlled drug release, maximizing therapeutic effects.
- Advanced Balloon Design: Provides excellent trackability, pushability, and crossability, even in challenging lesions.
Technical Specifications
- Balloon Material: Semi-compliant material for precise expansion and uniform drug delivery.
- Drug Coating: Paclitaxel, known for its anti-proliferative properties, to reduce neointimal hyperplasia.
- Size Options: Available in a variety of diameters and lengths to accommodate different lesion sizes and locations.
Clinical Benefits
- Effective Restenosis Prevention: Significantly reduces the risk of restenosis compared to standard PTA balloons.
- Improved Patient Outcomes: Enhances vessel patency and reduces the need for repeat interventions.
- Minimally Invasive: Offers a less invasive alternative to surgical interventions, promoting faster recovery times.
Clinical Study Overview
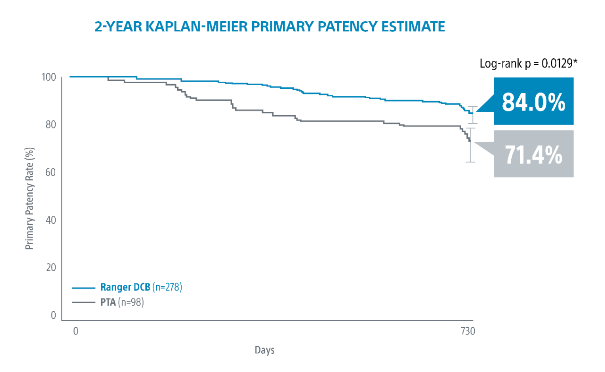
The Ranger™ Paclitaxel-Coated PTA Balloon Catheter has been extensively studied to demonstrate its safety and efficacy in treating PAD.
- Ranger SFA Trial: This randomized controlled trial evaluated the performance of the Ranger DCB in patients with superficial femoral artery (SFA) disease. Results showed a significant reduction in late lumen loss and target lesion revascularization (TLR) rates compared to uncoated balloons.

Sachar R, Soga Y, Ansari MM, Kozuki A, Lopez L, Brodmann M, Schroë H, Ramanath VS, Diaz-Cartelle J, Zeller T; RANGER II SFA Investigators. 1-Year Results From the RANGER II SFA Randomized Trial of the Ranger Drug-Coated Balloon. JACC Cardiovasc Interv. 2021 May 24;14(10):1123-1133. doi: 10.1016/j.jcin.2021.03.021. PMID: 34016410.